Faculty
-
Yoon, Jeong Kyo, Ph.D
Professor
Stem cell biology, Signaling pathways, Tissue regeneration, Skeletal muscle, Lung
Room 206, SIMS
+82-41-413-5016
jkyoon@sch.ac.kr
Yoon laboratory has a long-term research interest in understanding the molecular mechanisms by which tissue homeostasis and regeneration are regulated. We are currently studying two specific tissues, skeletal muscle and lung, by utilizing both in vitro cell culture and in vivo mouse models.
Skeletal Muscle
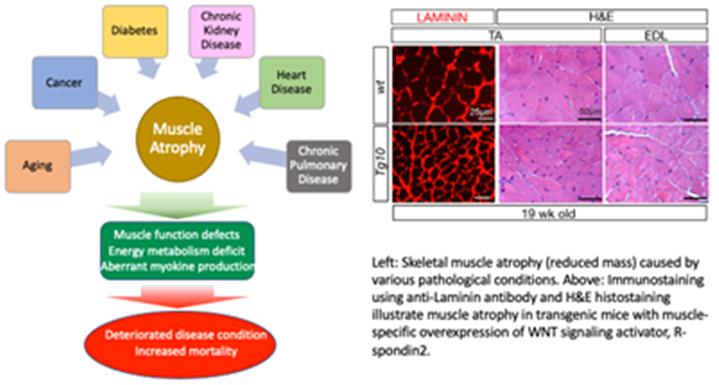
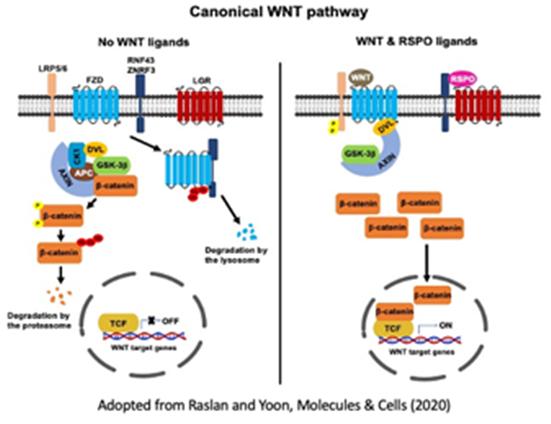
Skeletal muscle tissue primarily functions for body locomotion and energy metabolism and is a highly dynamic tissue that responds to various physiological changes such as physical damage, exercise, nutrition, aging, and disease conditions. During the regeneration process after muscle injury or exercise, muscle stem cells (also called satellite cells) residing within the skeletal muscle generate nascent myofibers for repairing the damaged muscle or building additional muscle mass. Independent from the stem cell activity, regulation of myofiber mass is another key cellular process for skeletal muscle homeostasis. A balance between catabolic and anabolic metabolism of cellular proteins and subcellular organelles is critical. We are investigating (1) the molecular mechanisms underlying stem cell quiescence, proliferation, and myogenic commitment, and (2) the novel signaling pathways that control skeletal muscle mass.
Lung
Lung tissue has a vital function in gas exchange between the blood and the external atmosphere. It also has a critical role in the immune defense against external pathogens and environmental factors. While the lung is classified as a relatively quiescent organ with little homeostatic turnover, it shows robust regenerative capacity in response to injury, mediated by the resident stem/progenitor cells. During regeneration, regionally distinct epithelial cell populations with specific functions are generated from several different types of stem/progenitor cells localized within four histologically distinguished regions: trachea, bronchi, bronchioles, and alveoli. WNT signaling is critical to its developmental role in the embryonic and fetal lung. We currently explore how WNT signaling regulates different types of stem/progenitor cells in the lung for regeneration.
|
Principal Investigator Biography B.S. in Zoology, Seoul National University, Korea M.S. in Zoology, Seoul National University, Korea Ph.D. in Mammalian Genetics, University of Illinois at Chicago, USA Research Fellow/ Senior Research Fellow, California Insititue of Technology, USA Faculty Scientist I/II, Maine Medical Center Research Institute, USA Associate Professor, Tufts University School of Medicine, USA Professor, Soonchunhyang Institute of Medi-bio Science(SIMS), Soonchunhyang University, Korea Research Interest - Signaling pathways in skeletal muscle regeneration and stem cells - Crosstalk between stem cells and other resident cells in skeletal muscle - Positive and negative regulators of skeletal muscle mass and their acting mechanisms - Regulation of stem cell function in lung regeneration Research Associates
Hyoshin Lee Bachelor of Science in Life Science and Biomedical Science, Hallym University, Republic of Korea Master of Science, Department of Integrated Biomedical Science, Soonchunhyang University. Republic of Korea Graduate Students
Jennifer Fransisca Bachelor of Science, Atma Jaya Catholic University, Indonesia Her research interest is to investigate about skeletal muscle niche components, particularly a group of mesenchymal progenitor cells called Fibro Adipogenic Progenitors (FAPs) that exhibit major impact in supporting muscle regeneration while become the main source of adipocyte and fibroblast infiltration in pathological muscle conditions. Bagus Sarmito Bachelor of Science, Atma Jaya Catholic University, Indonesia He is primarily interested in understanding the molecular mechanism by which skeletal muscle mass is regulated. Currently, he is studying a previously unknown role of chemokine CXCL14 in the regulation of skeletal muscle mass.
Youn Jeong Oh Bachelor of Science in Life Science, Hoseo University, Republic of Korea Master of Science in Biochemistry, Hoseo University, Republic of Korea Alumni Trinh Thi Tuyet Tran PhD Candidate, Nanyang Technological University, Singapore Nguyen Thi Thu Hao Postdoctoral Fellow, University of Texas Health Science Center at Houston, USA Hyunji Tak Research Associate, DT & CRO, Rebuplic of Korea Yeeun Kim Research Associate, DT & CRO, Rebuplic of Korea Ahmed Raslan Assistant Professor, Department of Zoology, Assiut University, Egypt Jay Prakash Shah Postdoctoral Fellow, University of Alabama at Birmingham, USA |




